

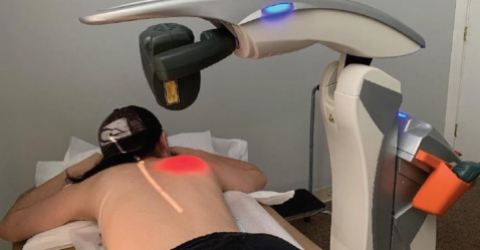



New opportunities to present ASA devices arrive from the United States, where the company, thanks to a stand set up by OrthoLazer Orthopedic Laser Centers (in cooperation with the local distributor Cutting Edge Laser Technologies), saw its M8 robotic device in the spotlight during the annual meeting of the American Association of Orthopedic Surgeons (AAOS).
“This meeting – explains Lucio Zaghetto, the President of ASA and Sales Manager for all of North America – was an important event for the industry in the years before the pandemic, attracting an average of 30,000 visitors. This year, there were around 6,000 visitors: there is no doubt that there has been a sharp drop in attendance compared to previous years, but the trend is certainly positive”.
This positive trend was also confirmed by the number of professionals who visited the OrthoLazer exhibition space, showing a real interest in learning more about the benefits of MLS® Laser Therapy and the uses of M8.



“We were able to discuss these uses also by meeting Dr. Scott Sigman, an orthopaedic surgeon from Massachusetts, founder of OrthoLazer and pioneer in the use of this device not only in his field. In fact, the luminary has also used it successfully to treat interstitial pneumonia caused by COVID-19 in a clinical trial in the United States. The patients affected by the virus, subjected to MLS® Laser Therapy saw significant improvements in several parameters including respiratory rates and blood oxygenation”.
ASA's participation at the Meeting in Chicago was therefore a success, also thanks to the growing interest of private equity companies specialising in the industry, interested in acquiring therapy centres and orthopaedic hospitals, for which to develop multi-year investment and growth plans.
“This possibility, which emerged during the 4th Annual Physicians Transactions Conference, organised as part of the AAOS event, if it takes shape, may certainly pave the way for new development channels that should not be underestimated”, concludes Zaghetto.






L'accesso alla visualizzazione dei prodotti e al materiale informativo è riservato agli operatori del settore in ottemperanza alla legislazione vigente. ASA richiede di qualificarsi come operatore del settore per procedere con la navigazione.
Decreto Legislativo 24 febbraio 1997, n°46 Articolo 21
1. E' vietata la pubblicità verso il pubblico dei dispositivi che, secondo disposizioni adottate con decreto del Ministro della Sanità, possono essere venduti soltanto su prescrizione medica o essere impiegati eventualmente con l'assistenza di un medico o di altro professionista sanitario.
2. La pubblicità presso il pubblico dei dispositivi diversi da quelli di cui al comma 1 è soggetta ad autorizzazione del Ministero della Sanità. Sulle domande di autorizzazione esprime parere la Commissione di esperti prevista dall'articolo 6, comma 3, del decreto legislativo
30 dicembre 1992, n. 541, che a tal fine è integrata da un rappresentante del Dipartimento del Ministero della Sanità competente in materia di dispositivi medici e da uno del Ministero dell'Industria, del commercio e dell'artigianato.
Some of the contents of this website cannot be disclosed in the USA and its territories and possesions, for regulatory reasons. If you are a US resident, please click on the button here below and access ASA's distributor website for North America.