ASA Training Experience: two days of applied laser therapy for rehabilitation professionals
Two immersive days of advanced clinical training as a privileged meeting point for health professionals interested in learning more about the use of Hilterapia® and MLS® Laser Therapy in therapeutic pathways. The intensive course organised in Pavia by ASA in collaboration with the Mediperson Medical Centre, the venue of the event, confirmed the value of the “Travel & Learn” formula: a complete, practical and stimulating educational experience designed to promote active and engaging learning.
Two days of technology and clinical application
Held on 9 and 10 June 2025, the course welcomed physiotherapists and rehabilitation professionals from the Czech Republic, the United Kingdom, Romania, Austria, Hungary and Australia with the aim of offering not only an in-depth theoretical overview of the ASA technologies, but also a practical focus on real clinical cases. The hands-on sessions, led by Salvatore Germano and Mata Karagianni, strengthened the understanding of the therapeutic potential of M-Hi and HIRO, highlighting how these technologies effectively integrate into the management of musculoskeletal disorders and into complex rehabilitation programs.

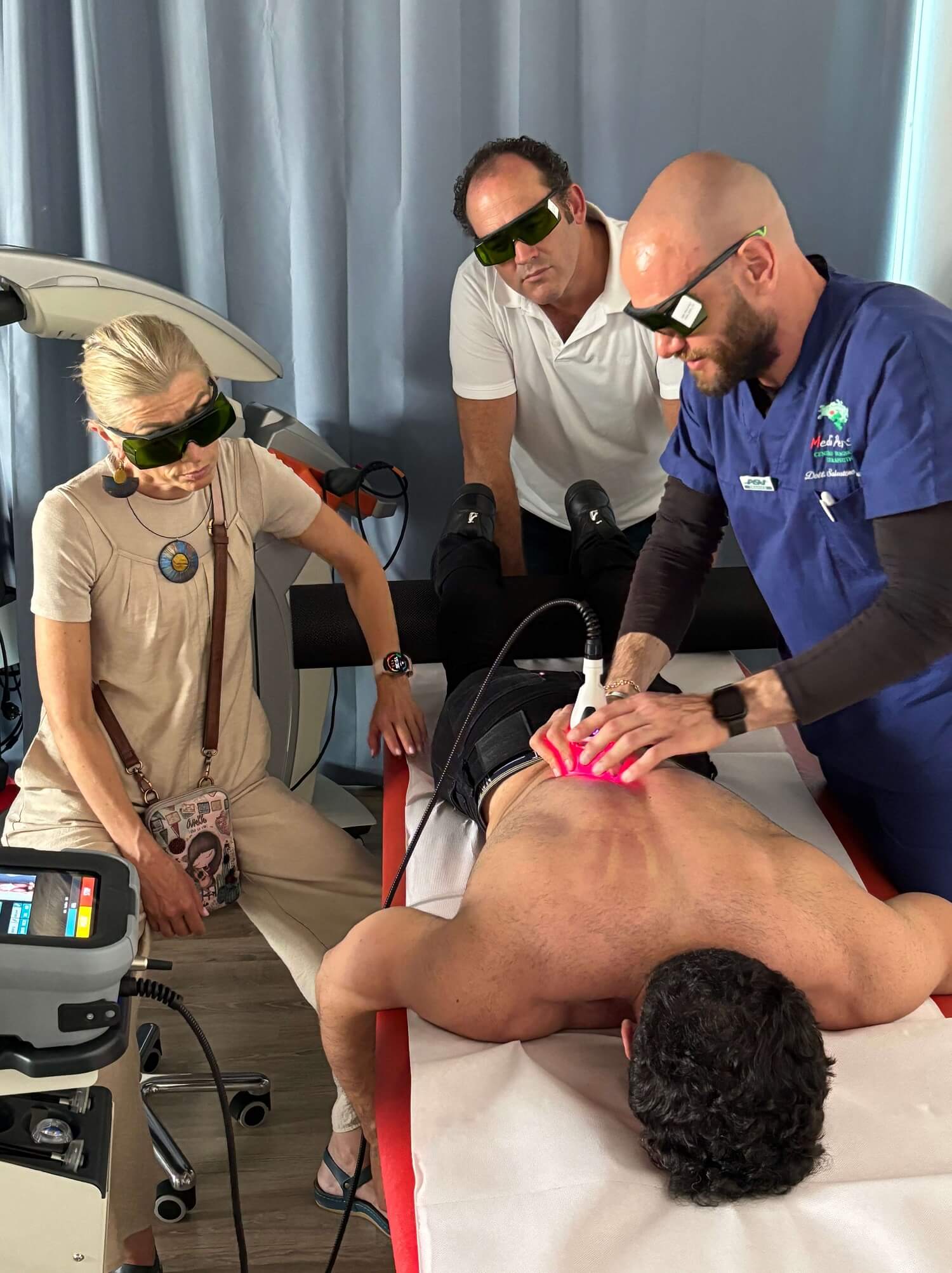



ASA: where innovation meets training excellence
The activity is part of ASA's broader training program, aimed at offering healthcare professionals not only excellent technological tools, but also solid clinical skills, which allow an informed and strategic use of laser therapy. The ability to combine advanced technology and high-level training is what distinguishes ASA as a leader in the supply of medical devices and in the competence of its customers.
Feedback from those who experienced the course first-hand

“It was really helpful to see such a different practical approach than the usual one: the use of laser combined with massage... and then working on real patients made all the difference.”
- Kirsten Sinclair

“Being able to use the devices directly, understanding how to customise therapies without being limited to standard protocols, and exchanging ideas even during breaks... was super stimulating.”
- Andras Gyemant
Would you like to have this experience too?
Discover the upcoming scheduled events and join the community of professionals who choose ASA to grow, innovate and improve the quality of their daily work.











