



Osteoporosis is recognised as a major public health problem due to its close association with fractures in individuals over the age of 50, with potentially serious physical and psychological consequences and an associated increased risk of death.
In particular, hemiparetic patients are at high risk of developing osteoporosis, and are more predisposed to a higher risk of falls and fractures.
Although medications currently represent the main point of reference for the treatment of osteoporosis, it is important to note that they can lead to side effects, especially if used long-term. Therefore, therapeutic alternatives to improve clinical outcomes and limit the use of medications are constantly sought after. Physical activity and the application of physical therapies are showing promising results in this context.
Abo Elyazed and colleagues, in a recent randomised controlled trial, published in the Journal of Orthopaedic Surgery and Research (2023), explored and compared the effects of high intensity laser therapy (HILT) and Extracorporeal Shockwave therapy (ESWT), in combination with pharmacological therapy and physical exercise, in hemiparetic patients suffering from osteoporosis.
The research could help address the rehabilitation of these patients in an increasingly effective manner.
120 patients suffering from osteoporosis/osteopenia were divided at random into three groups:
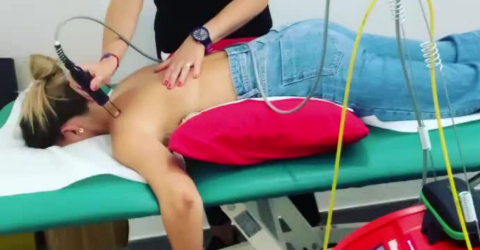
The treatments, laser therapy and shock waves, were administered three times a week for 12 weeks, for a total of 36 sessions. The lumbar and hip areas were treated.
The laser therapy sessions used the ASA - Hiro 3 device from the Hilterapia® family.
The following were assessed for each patient before and after the treatment cycle:
The intergroup analyses highlighted significant variations in all the outcomes investigated in all three groups.
Post-treatment comparisons highlighted significant differences between the three groups. In particular, the HILT and ESWT groups showed more marked improvements than the control group in all the assessments performed (VAS, General Stability Index, SFBBS and QUALEFFO-41).
In terms of risk of falls (General Stability Index and SFBBS) and quality of life (QUALEFFO-41), a significant difference was found in favour of HILT (Hilterapia®) compared to ESWT. HILT and ESWT were proved to be equally effective in reducing pain.
The results of this study are promising and demonstrate how the addition of Hilterapia® or ESWT to standard therapy - with greater evidence in favour of Hilterapia® - leads to clinically significant improvements in the treatment of hemiparetic patients with osteoporosis.





L'accesso alla visualizzazione dei prodotti e al materiale informativo è riservato agli operatori del settore in ottemperanza alla legislazione vigente. ASA richiede di qualificarsi come operatore del settore per procedere con la navigazione.
Decreto Legislativo 24 febbraio 1997, n°46 Articolo 21
1. E' vietata la pubblicità verso il pubblico dei dispositivi che, secondo disposizioni adottate con decreto del Ministro della Sanità, possono essere venduti soltanto su prescrizione medica o essere impiegati eventualmente con l'assistenza di un medico o di altro professionista sanitario.
2. La pubblicità presso il pubblico dei dispositivi diversi da quelli di cui al comma 1 è soggetta ad autorizzazione del Ministero della Sanità. Sulle domande di autorizzazione esprime parere la Commissione di esperti prevista dall'articolo 6, comma 3, del decreto legislativo
30 dicembre 1992, n. 541, che a tal fine è integrata da un rappresentante del Dipartimento del Ministero della Sanità competente in materia di dispositivi medici e da uno del Ministero dell'Industria, del commercio e dell'artigianato.
Some of the contents of this website cannot be disclosed in the USA and its territories and possesions, for regulatory reasons. If you are a US resident, please click on the button here below and access ASA's distributor website for North America.