





In occasion of the 16th European Congress of Physical and Rehabilitation Medicine, Bruges, Belgium Professor Raoul Saggini delivered a speech about a study sourced from his cooperation with ASAcampus; the study contributes to explain the combined effect of Hilterapia® at a clinical level with the effect of the pulsed Nd:YAG laser (HILT®) on cells.
HILT®-THERAPY IN «PAINFUL SHOULDER» SYNDROME FROM PARTIAL TEAR AND CALCIFIC TENDINOPATHY OF THE ROTATOR CUFF. EFFECTS AT CELLULAR LEVEL
Saggini R.1 and Monici M.2
1) Physical and Rehabilitative Medicine, G.DʼAnnunzio University, Chieti, Italy
2) ASAcampus-ASA Research Division, Dept. of Clinical Physiopathology, University of Florence, V.le Pieraccini 6, I-50139 Florence, Italy
Introduction - The objective of the different kinds of treatments in the «painful shoulder syndrome» is not the complete restoration of the cup, but rather a functional as well as structural recovery from the inflammatory and degenerative conditions of the tissues, that can constitute an aggravating factor in the evolution of the disease, painfulness, as well as functional disability. Recently, treatments based on mechanical stimulation by devices which deliver physical energy to the tissue are regarded with growing interest.
Aim - i) To evaluate the therapeutic impact of tissue stimulation due to physical energy delivering by Nd:YAG laser pulses (HILT®-Therapy); ii) to compare the efficacy of HILT®-Therapy with high-energy shock-waves, a first-level treatment for the considered disease; iii) to establish a relationship between the efficacy of HILT®-Therapy and the effects at cellular level.
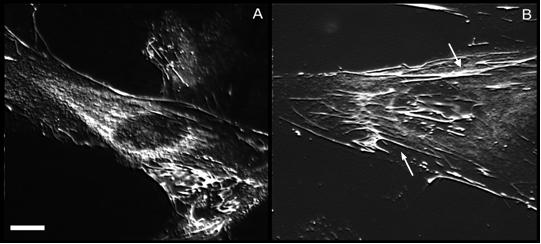
Patients and Methods - 40 subjects were treated and clinically controlled for a period of 360 days. We randomly formed two groups of 20 units homogenous for sex and age, treated consecutively with two different methods of physical energy: the former (group A) with 10 sessions of HILT®-Therapy; in the latter (group B) a Spark Gap Electro-hydraulic HMT Evotron was used. Moreover, in both the groups of patients, passive and active kinesitherapy was associated. The effects at cellular level were studied on cell cultures treated by pulsed Nd:YAG laser. Cell response was analysed by immunofluorescence, autofluorescence and PCR techniques
Results — We monitored net and lasting benefit induced by HILT®-Therapy (85% patients), comparable with the clear and lasting benefit obtained with shock-wave treatment (80% patients). The efficacy of HILT®-Therapy can be explained with mechanical stimulation of tissues, mainly due to photomechanical stress. In fact, changes in cell energy metabolism, cytoskeleton organization, production of extracellular matrix components have been observed in cell cultures.
Conclusion - Based on our experience of clinical and instrumental observation, we can conclude that it is possible to codify a routine approach in the therapeutic-rehabilitative course in the case of incomplete lesions of the rotator cuff through the integrated use of physical energies, such as HILT®-Therapy, in association with rehabilitation exercises.






L'accesso alla visualizzazione dei prodotti e al materiale informativo è riservato agli operatori del settore in ottemperanza alla legislazione vigente. ASA richiede di qualificarsi come operatore del settore per procedere con la navigazione.
Decreto Legislativo 24 febbraio 1997, n°46 Articolo 21
1. E' vietata la pubblicità verso il pubblico dei dispositivi che, secondo disposizioni adottate con decreto del Ministro della Sanità, possono essere venduti soltanto su prescrizione medica o essere impiegati eventualmente con l'assistenza di un medico o di altro professionista sanitario.
2. La pubblicità presso il pubblico dei dispositivi diversi da quelli di cui al comma 1 è soggetta ad autorizzazione del Ministero della Sanità. Sulle domande di autorizzazione esprime parere la Commissione di esperti prevista dall'articolo 6, comma 3, del decreto legislativo
30 dicembre 1992, n. 541, che a tal fine è integrata da un rappresentante del Dipartimento del Ministero della Sanità competente in materia di dispositivi medici e da uno del Ministero dell'Industria, del commercio e dell'artigianato.
Some of the contents of this website cannot be disclosed in the USA and its territories and possesions, for regulatory reasons. If you are a US resident, please click on the button here below and access ASA's distributor website for North America.