





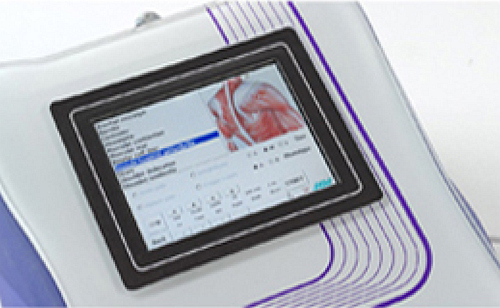
His name is Mphi* and it will be available from March 2010. It is the new portable device for MLS® Laser Therapy with handpiece , which will allow an easy and functional use of this exclusive therapy in many situations. In fact this new device is light and compact, it can easily be transported over the shoulder and works also with battery. The laser emission is the proven MLS® pulse, which revolutionized the diode laser therapy thanks to a quality approach oriented to the best therapeutic result. Through a modern colour touch-screen display user gets access to a completely new software that offers a new approach of use with four different treatment modalities and with the possibility of adapting the parameters to the patient’s characteristics.
La “teaching machine” for MLS® Laser Therapy
With its renewed interface logic, the Mphi touch-screen was developed to come closer to the personal therapeutic approach of the operator: 3 different use modes in “Pain management”, and another 2 for treating oedema and for biostimulation (injuries, ulcers, sores). The new software gives different treatment personalisation according to the “phototype” and “dimensions” of the patient: it is also possible to select “acute” or “chronic” values.
* The name Mphi is ispired by the golden ratio, symbolized by the greek letter PHI (Φ).
The golden ratio indicates a proportion used to make more beautiful and proportioned architectural buildings: the Parthenon of Athens was built according to this proportion and the PHI symbol was born just at that time, in honour of Fidias (Φειδίας), the designer of Parthenon. Applied to numerous architectural and artistic works in all ages, from the Cheope's Pyramid to da Vinci's Mona Lisa and, in more recent times, to the Modulor by Le Corbusier, now PHI becomes also the symbol of the harmony between the two wavelengths which compose the MLS® pulse.










L'accesso alla visualizzazione dei prodotti e al materiale informativo è riservato agli operatori del settore in ottemperanza alla legislazione vigente. ASA richiede di qualificarsi come operatore del settore per procedere con la navigazione.
Decreto Legislativo 24 febbraio 1997, n°46 Articolo 21
1. E' vietata la pubblicità verso il pubblico dei dispositivi che, secondo disposizioni adottate con decreto del Ministro della Sanità, possono essere venduti soltanto su prescrizione medica o essere impiegati eventualmente con l'assistenza di un medico o di altro professionista sanitario.
2. La pubblicità presso il pubblico dei dispositivi diversi da quelli di cui al comma 1 è soggetta ad autorizzazione del Ministero della Sanità. Sulle domande di autorizzazione esprime parere la Commissione di esperti prevista dall'articolo 6, comma 3, del decreto legislativo
30 dicembre 1992, n. 541, che a tal fine è integrata da un rappresentante del Dipartimento del Ministero della Sanità competente in materia di dispositivi medici e da uno del Ministero dell'Industria, del commercio e dell'artigianato.
Some of the contents of this website cannot be disclosed in the USA and its territories and possesions, for regulatory reasons. If you are a US resident, please click on the button here below and access ASA's distributor website for North America.